As pharmaceutical prices started to climb in the early 1990s, the federal government passed legislation to allow certain categories of non-profit, health care facilities – called “covered entities” – that served primarily underserved or disadvantaged populations to receive discounts from pharmaceutical manufacturers. This drug discount program is commonly referred to as the 340B Program and is overseen by the Health Resources and Services Administration (HRSA).
Manufacturers are often required to adjust the 340B price of a drug based on various factors, including the non-340B drug cost and statutory formulas. Prior to 2019, however, there was little oversight into whether manufacturers really were offering the correct 340B drug price to covered entities. Now, to avoid legal sanctions, manufacturers must publicly notify covered entities if the 340B price formula has resulted in the reduction of a drug’s 340B price such that the covered entity is entitled to a refund. While these public notifications of 340B drug price corrections increase transparency, they can also lead to covered entities not having full access to the discounts to which they are entitled.
Background
On April 1, 2019, HRSA launched a new ceiling price website for the 340B Program. This website outlines the maximum price (the “ceiling price”) that a pharmaceutical manufacturer can charge for any of its drugs when the drug is part of the 340B Program. HRSA created the ceiling price website to increase 340B drug pricing transparency and to facilitate the implementation of new 340B regulations1 that impose a civil monetary penalty on any pharmaceutical manufacturer that knowingly or intentionally charges a 340B covered entity a price greater than the ceiling price for 340B covered drugs.
To give pharmaceutical manufacturers an opportunity to correct any overcharges before facing enforcement actions, HRSA’s ceiling price website also created a location for pharmaceutical manufacturers to post their “notices of correction.” These notices are publicly available and are intended to inform 340B covered entities of specific drugs that the pharmaceutical manufacturer overcharged for (that is, charged a price greater than the ceiling price). The notices then advise the 340B covered entities either that they will automatically receive a refund in the amount of the overcharge or that they can request a refund of the overcharge amount from the pharmaceutical manufacturers. Most notices of correction contain multiple drugs that the pharmaceutical manufacturer overcharged for during a particular time period.
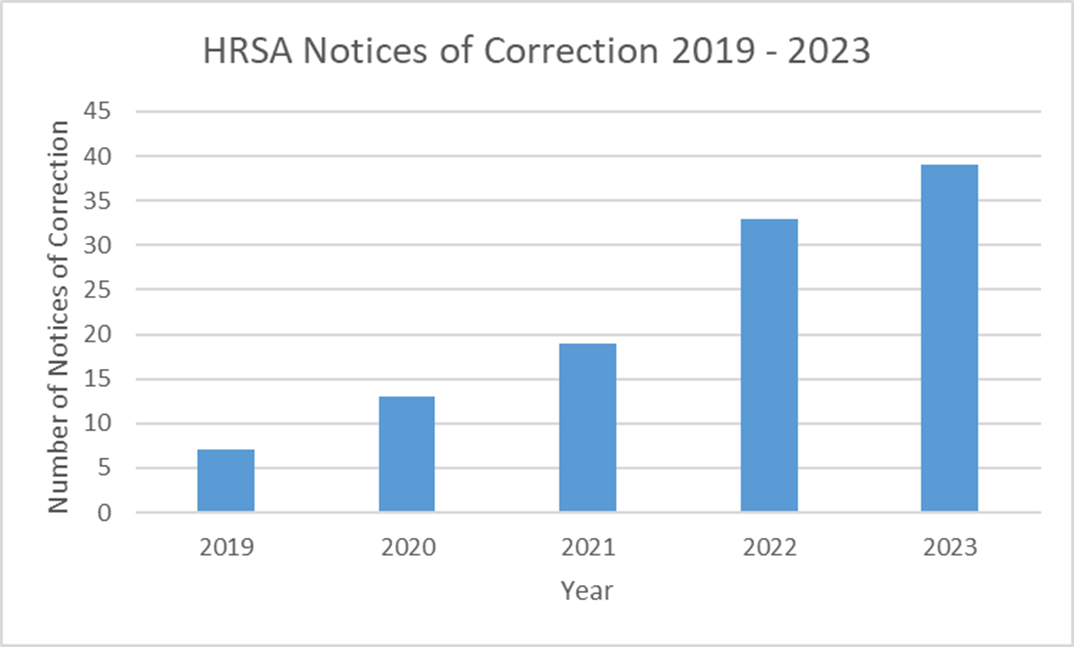
Prior to the creation of HRSA’s 340B ceiling price website in 2019, only 13 total pharmaceutical manufacturer notices of correction had been issued as part of the 340B program since its inception in 1992. That number has since skyrocketed. From 2019 through 2023, since the ceiling price website went live, there have been 111 total notices of correction.
Since 2019, the following pharmaceutical manufacturers have posted seven or more notices of correction: Eli Lilly, GlaxoSmithKline, Johnson & Johnson, Merck & Co., Novo Nordisk, and Purdue Pharmaceuticals. The notices generally cover the time period that the ceiling price website has been active; they range from Q1 2019 through Q4 2023. Additionally, many of these major pharmaceutical manufacturers repeatedly issue notices of correction for the same drug. For example, Novo Nordisk has issued three notices of correction for Ozempic since 2022; Eli Lilly has issued five for Cialis and six for Strattera since 2021.
It is common for pharmaceutical manufacturers to impose “refund limitations” that require 340B covered entities to take action in order to receive the refunds they are owed. For example, GlaxoSmithKline will only automatically issue refunds to covered entities that are owed an aggregate refund of $100 or more; all covered entities that are owed less than $100 must contact GlaxoSmithKline to request a refund. While many of the pharmaceutical manufacturer refund limitations are relatively small (e.g., $10 or less, $50 or less), some manufacturers impose an automatic refund limitation of $1,000 or less in aggregate overpayments while others will not automatically issue a refund at all, regardless of the covered entity’s amount of aggregate overpayment.
What does this mean? It means that, on an ongoing basis, some covered entities may not be realizing the full benefit of the 340B discounts they are entitled to under law.
At least one covered entity is even making the case that manufacturers were not complying with ceiling price requirements before HRSA’s ceiling price website went live in 2019. In U.S. ex rel Adventist Health Sys., et al. v. AbbVie, Inc., et al., Adventist Health System, a 340B covered entity, is suing pharmaceutical manufacturers AbbVie, AstraZeneca, Novartis, Sanofi, and Sandoz alleging that the manufacturers caused violations of the False Claims Act and various state anti-fraud laws when calculating the 340B price for drugs with price increases that outpaced inflation. Adventist Health is seeking hundreds of millions of dollars in damages and penalties from the manufacturers in this whistleblower lawsuit.
Looking Forward
What can covered entities do? To ensure that they are not overpaying for 340B drugs and are receiving the full amount of any refund owed, covered entities can take the following action steps:
- Check HRSA’s website regularly. All notices of correction are posted and maintained on HRSA’s ceiling price website. We recommend visiting this website monthly and reviewing the newly posted notices of correction to determine refund eligibility and dates after which refunds will no longer be issued. Covered entities can also create an email alert for when new notices of correction are posted.
- Maintain consistent records of 340B drug purchasing. Maintaining 340B purchasing records in a manner that allows searching by NDC and drug name, broken down by purchase quarter, can help a covered entity quickly determine whether a particular notice of correction applies to their purchases and the amount of refund owed.
- Contact the pharmaceutical manufacturer. If a refund is not received, the covered entity should contact the pharmaceutical manufacturer and request that they send a refund.
This blog post was drafted by Beth Siemer and Aurora Kammerer, attorneys in the St. Louis, Missouri, and Overland Park, Kansas, offices of Spencer Fane, respectively. For more information, visit www.spencerfane.com.
1 42 C.F.R. 10.10; 10.11; see also 82 F.R. 1210.

